Warmest Congratulations!
With remarkable achievements in two major fields—inhaled drug delivery devices and injection pens, Suzhou Senbomed Medical Technology Co., Ltd. has emerged as a brilliant new star, surging ahead of the competition and earning prestigious recognition as a National High-Tech Enterprise. This honor is undoubtedly a high-profile endorsement and top-tier accolade for Senbo’s commitment to deepening expertise in the drug delivery device sector and its relentless pursuit of exceptional quality.
About Senbomed Medical——A Dual Champion in Inhaled & Injectable Drug Delivery
Specializing in cutting-edge R&D, Senbomed Medical is a leading provider of one-stop solutions for drug delivery devices. Its business scope covers key areas including inhaled drug delivery devices, micro-nebulization drug delivery devices, and R&D of injection pens.
Benefiting from the team’s profound industry experience, superior product quality, and sophisticated technical capabilities, Senbomed has achieved rapid growth in both inhaled and injectable drug delivery segments within just two years of its establishment. Exceeding industry expectations, the company has secured orders from many leading pharmaceutical and biological companies, winning extensive market recognition and acclaim. It stands as a rare industry leader excelling in two core drug delivery fields simultaneously.
Notably, within less than two years of its founding, Senbomed was invited to participate in the drafting of the 2025 edition of the Pharmacopoeia standards—a testament to its outstanding technical expertise and robust comprehensive strength.
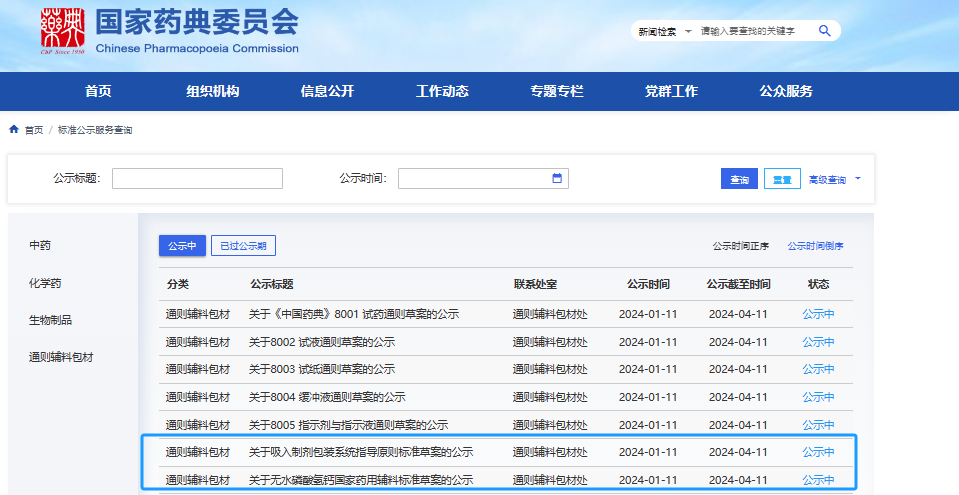
The national pharmacopoeia standard Packaging Systems for Inhalation Preparations, for which Senbomed Medical participated in the drafting, has completed the public consultation period.
One-Stop Turnkey Engineering Solutions
Senbomed Medical has assembled a team of top-tier professionals specializing in inhaled drug delivery devices and auto-injectors, who are fully dedicated to rigorous R&D and innovation.
Backed by cutting-edge technological strength and an unwavering commitment to craftsmanship excellence, Senbomed boasts a mature and comprehensive technical workflow that covers the entire product lifecycle. This integrated process encompasses everything from the initial spark of product design concepts, precision structural engineering, and strict tolerance analysis, to careful material selection, multiphase flow simulation analysis, precision mold development, process validation, high-precision injection molding, product verification, and final assembly.
Leveraging this full-fledged capability, Senbomed is well-positioned to deliver customized, integrated, and high-quality professional solutions tailored to meet the unique needs of each client.


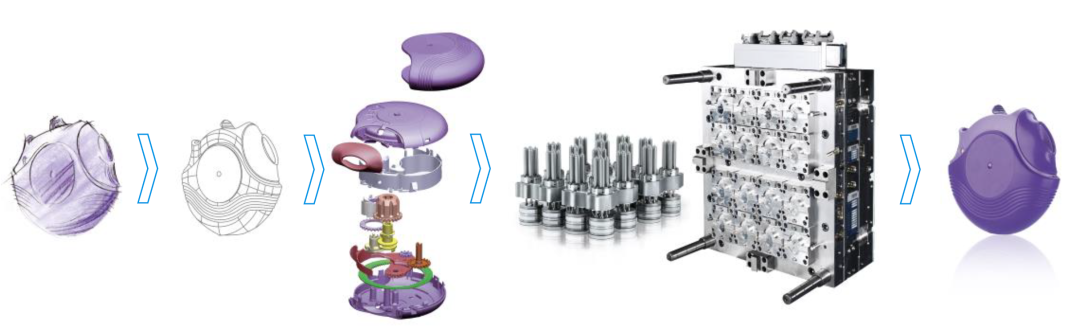
(Taking the entire lifecycle of Project 2201 as an example)
World-Class Equipment
Backed by the top-tier academic resources of academicians from the Monash-SEU Joint Research Institute and robust supercomputing power support, Senbomed Medical has independently established an in vitro bioequivalence evaluation laboratory for inhalation preparations and drug delivery devices, which fuels continuous innovation and R&D initiatives.
Its industrial manufacturing center, located in Suzhou High-tech Zone, houses a 2,600-square-meter Class 100,000 cGMP cleanroom. Equipped with world-class production and testing equipment, the center features specialized labs for microbial testing, optical inspection, and physicochemical property analysis. Capable of conducting precise tests in accordance with United States Pharmacopeia (USP) standards and China National Pharmaceutical Packaging Material (YBB) standards, the manufacturing process is fully compliant with both pharmaceutical packaging and medical device regulations. This ensures every single product delivers superior, reliable quality.
Partial equipment and instrument display

R&D team
Senbomed Medical’s device R&D team is an elite force in the industry. For generic drug delivery devices, the team leverages sophisticated patent circumvention expertise to skillfully resolve intellectual property challenges. For innovative drug delivery devices, it demonstrates exceptional design ingenuity and patent filing capabilities, translating bold ideas into granted patents at maximum speed.
This seasoned team has supported numerous domestic pharmaceutical listed companies in successfully developing a full range of self-administered drug delivery devices, including multi-dose blister inhalers, single-dose capsule inhalers, soft mist inhalers, nasal spray devices, ophthalmic micro-nebulization delivery devices, and auto-injectors. These devices are widely used across multiple critical medical fields, such as asthma, COPD (Chronic Obstructive Pulmonary Disease), nasal disorders, ophthalmic conditions, immunology, and endocrine therapies for diabetes and weight management.
Throughout the device development process, the team has faced numerous challenges, yet it has always maintained composure and focus. By fostering seamless communication and collaboration, the team has overcome one technical hurdle after another, creating a portfolio of market-leading oral inhalation devices and premium auto-injectors that have wowed the industry. Every meticulous detail embodies the team’s earnest aspiration to help patients regain health; every innovative breakthrough is dedicated to empowering medical professionals with more efficient treatment tools.
Partial Achievements
-
All devices are validated and fully bioequivalent to the reference listed drugs, enabling direct substitution.
-
All devices support customized exterior design to meet specific brand requirements.
-
Devices can be integrated with custom sensors, which sync with mobile apps to send medication reminders and monitor patients’ inhalation flow rate and duration.
-
100% in-house manufacturing ensures full control over both product quality and production costs.

Soft mist drug delivery device

Dual-Blister Dry Powder Inhaler (similar to Ellipta)

Auto injector (1ml PFS) (DMF 040385)

Spring Powered Disposable multi-dose injection pen
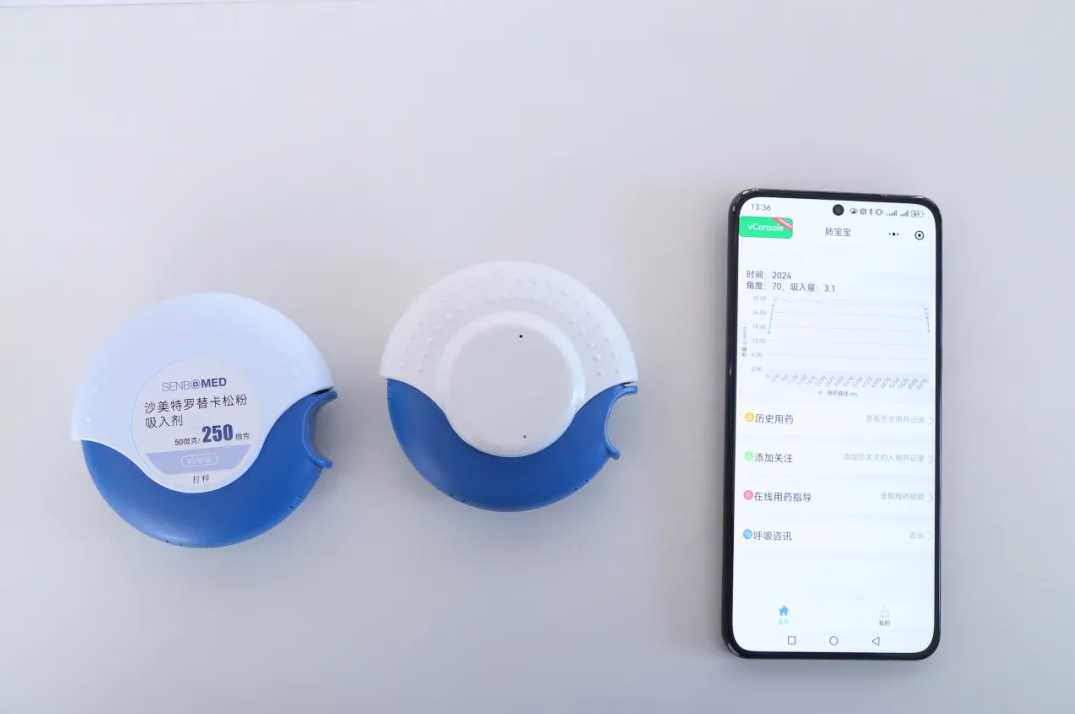
Imitation of Seretide Drug Delivery Device&Intelligent Inhalation Drug Delivery Monitoring Device

Capsule type dry powder delivery device (similar to Breezhaler)

Capsule type dry powder delivery device (imitating Handihaler)
Precision Mould & Injection Molding Team
Senbomed Medical boasts a team specialized in developing plastic moulds and flash-free liquid silicone rubber (LSR) moulds, with core members hailing from European industry backgrounds. Our team provides robust technical support for the entire injection molding production process.
Our in-house mould design team focuses exclusively on the design and development of precision moulds for drug delivery devices. Complementing this, the seasoned in-house mould assembly team not only assembles the precision-machined mould components, but also excels in optimizing the final mile of the mould manufacturing process. This meticulous approach significantly enhances mould precision and stability, laying a solid foundation for efficient, consistent mould-based production.
To ensure uninterrupted, stable manufacturing, Senbo Medical (Suzhou) is equipped with world-class, fully servo-driven electric injection molding machines imported from Japan. These machines feature closed-loop control systems that guarantee consistent performance across different moulds, providing an additional safeguard for sustained, high-quality production.
Since its establishment, Senbomed Medical has adhered to the philosophy of scientific injection molding. All molding process development is carried out in strict accordance with internationally recognized scientific injection molding methodologies.

Quality Assurance Team
While ensuring solid manufacturing fundamentals, Suzhou Senbomed Medical Technology Co., Ltd. has built a robust quality assurance team, bringing onboard seasoned quality professionals with work experience at European companies. Leveraging audits against the ISO 13485 quality management system, the company consistently delivers constructive training to nurture its talent pool. Since its inception, Senbomed has been committed to establishing a high-caliber, industry-leading quality management system.
In terms of compliance with standards, Senbomed adheres to stringent criteria at all times. The drug delivery devices it R&Ds and manufactures are fully aligned with the relevant inhaled drug product evaluation guidelines issued by the U.S. FDA. Moreover, the entire lifecycle—from initial R&D to end-of-supply-chain processes—strictly complies with the ISO 13485 medical device design and manufacturing standard. This all-round, zero-blind-spot approach ensures the quality and safety of every product.





















